Redesign and engineering of the autotransporter β-barrel domain
This project will examine the structures and folding behaviour of autotransporter proteins and reengineered derivatives fused to target heterologous proteins. This will provide insights into factors that dictate their folding and enhance the production of recombinant proteins for research and commercial needs.
Research themes
Project status
Content navigation
About
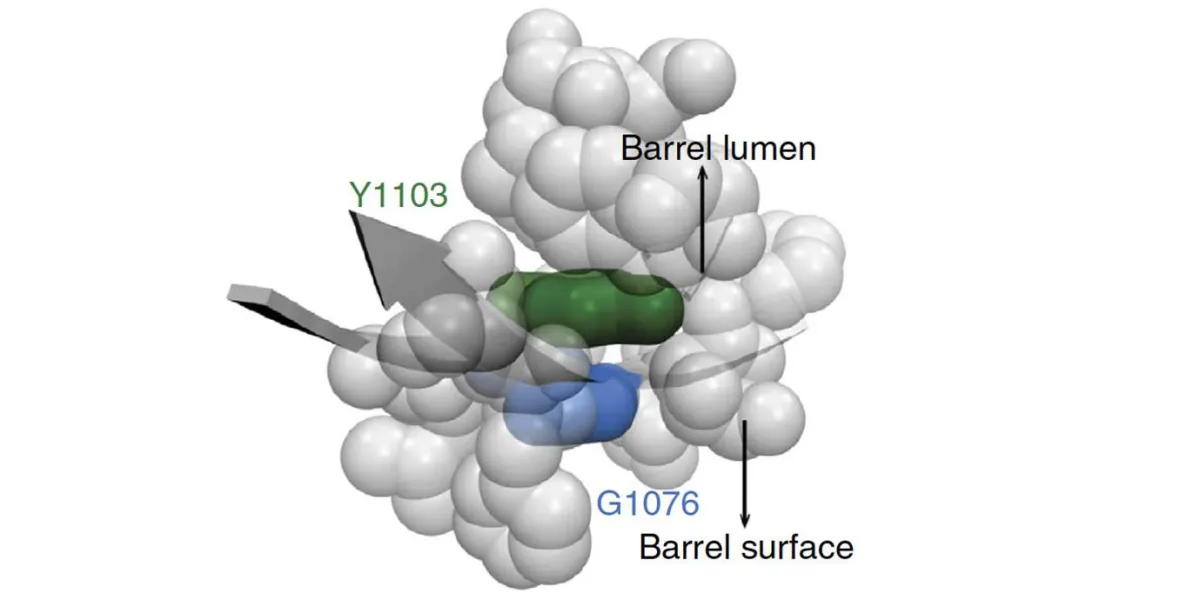
We have exploited the modular structure of autotransporters and their biogenesis pathway to secrete folded heterologous recombinant proteins into the culture medium through substitution of the native passenger domain with the heterologous protein of interest. The rapid secretion of recombinant proteins with functional, structural and size heterogeneity, as well as multicomponent complexes suggests that the autotransporter β-barrel domain serves as a generalised folding device by acting as a structural template for rapid nucleation of both native and heterologous passenger domain folding. Therefore, a third objective of my lab is to dissect the role of the β-barrel domain in the folding of both native and heterologous passenger domains. In collaboration with Assoc/Prof Ashley Buckle at Monash University (http://pxgrid.med.monash.edu.au/projects), we will use this knowledge to guide the redesign and engineering of the autotransporter β-barrel domain for enhanced folding properties. It is expected that this work will lead to the generation of reengineered β-barrel variants with enhanced folding properties that will serve to enhance recombinant protein production to meet a spectrum of research and commercial needs.
This project will suit students with an interest in recombinant protein production, protein design and engineering, and structural biology. Training will be provided in molecular biology, protein biochemistry, protein purification, protein refolding in detergent and in a variety of lipid systems, protein biophysics, protein design and engineering, and structural biology of membrane proteins. Further details may be obtained from Dr Leyton.

