To what degree does autotransporter folding inside the bacterial cell resemble autotransporter folding in bulk solution?
Autotransporters are synthesised in the cytoplasm with a specific domain architecture that confers a unique transmembrane topology once assembled into bacterial outer membranes.
Research themes
Project status
Content navigation
About
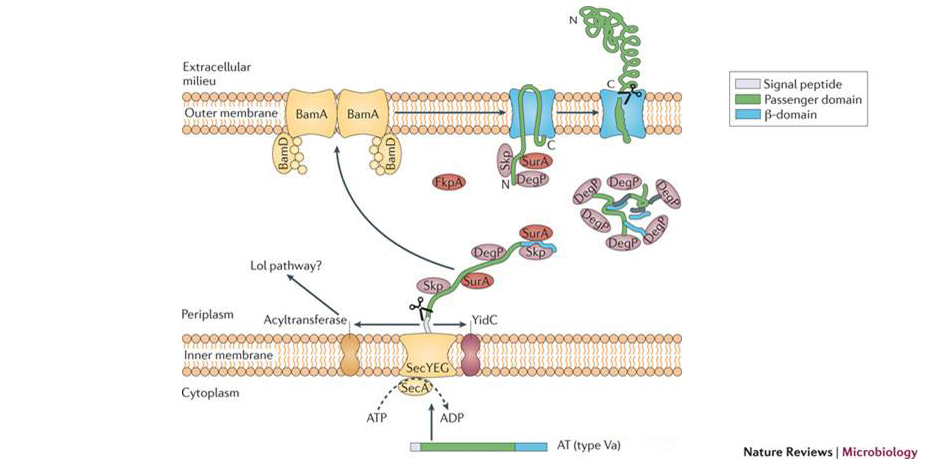
Autotransporters are synthesised in the cytoplasm with a specific domain architecture that confers a unique transmembrane topology once assembled into bacterial outer membranes. An N-terminal signal peptide directs secretion of the protein across the inner membrane, and a domain composed of a 12-stranded β-barrel is then folded and embedded in the outer membrane to facilitate presentation of the extracellular (passenger) domain to the bacterial cell surface. Once translocated, some passenger domains remain attached to their β-barrels to function as adhesins or invasins while others are processed and released into the environment to perform other effector functions. We and others have shown that the biogenesis of autotransporters is entirely dependent on a set of key molecules that, in a cellular context, catalyse the steps in their transport and assembly, as they do for other types of β-barrel proteins. We recently reconstituted autotransporter folding in vitro, showing for the first time that autotransporters are able to fold efficiently in the absence of accessory molecules through an intrinsic folding pathway of unknown mechanism. This observation raised the important question of why autotransporters require accessory molecules to fold into bacterial outer membranes. As such, a second objective of my lab is to address this question by comparing to what degree autotransporter folding inside the bacterial cell resembles autotransporter folding in bulk solution. This work is carried out in collaboration with Prof Ian Henderson at the University of Birmingham. It is expected that the outcomes of this work will reveal ways to interfere with the folding process and thereby drive discovery of innovative strategies to inhibit autotransporter function as an additional means to prevent autotransporter-mediated infection and disease.
This project will suit students with an interest in protein folding and membrane transport processes. Training will be provided in molecular biology, protein biochemistry, protein purification, protein refolding in detergent and in a variety of lipid systems, and protein biophysics. Further details may be obtained from Dr Leyton.

