G protein-coupled receptors: the structural basis for their pharmacology
Speakers
Event series
Content navigation
Description
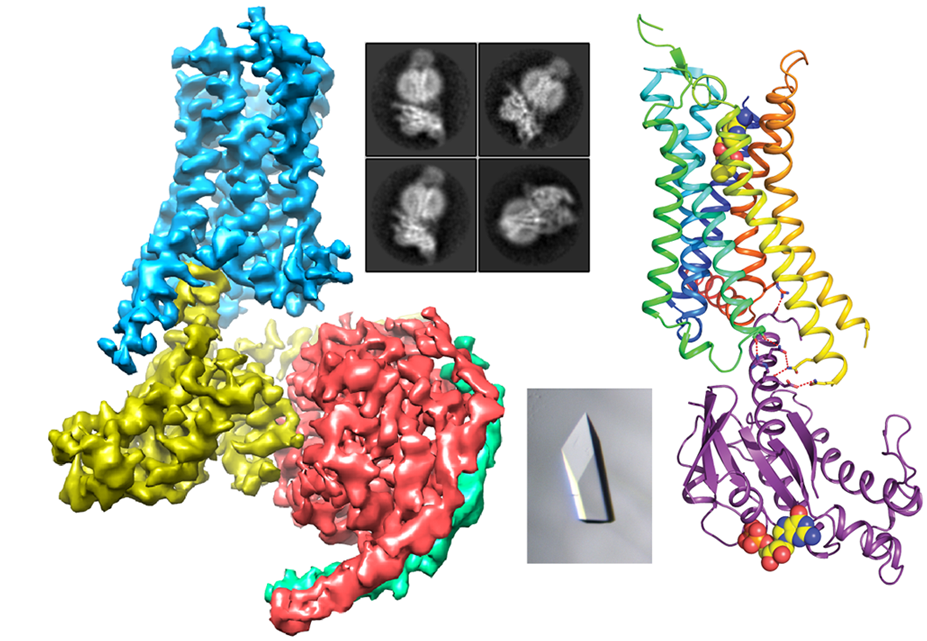
G protein-coupled receptors (GPCRs) activate intracellular signalling proteins (G proteins and arrestins) in response to extracellular signalling molecules. GPCRs are highly dynamic proteins, which rapidly interchange between different conformational states. In order to understand the molecular mechanisms of GPCR activation, structures of GPCRs in different conformational states are required.
We have determined structures of the adenosine A2a receptor (A2aR) and b1 adrenoceptor (b1AR) in a variety of different states bound to ligands ranging from inverse agonists to full agonists. Structures in active states have also been determined of A2aR and the serotonin 5-HT1B receptor coupled to the heterotrimeric G proteins Gs and Go, respectively. I will use this wealth of structural data to address a number of questions with respect to GPCR pharmacology such as why G proteins cause an increase in agonist affinity and what are the factors that affect the specificity of G protein coupling.
- García-Nafría, J., Lee, Y., Bai, X., Carpenter, B. & Tate, C.G. (2018) Cryo-EM structure of the adenosine A2A receptor coupled to an engineered heterotrimeric G protein. eLife 7, e35946
- García-Nafría, J., Nehmé, R., Edwards, P.C. & Tate, C.G. (2018) Cryo-EM structure of the serotonin 5-HT1B receptor coupled to heterotrimeric Go. Nature, In press.
- Warne, T., Edwards, P.C. Doré, A.S., Leslie, A.G.W. & Tate, C.G. (2018) Molecular diversity of high affinity agonist binding in active states of the β1-adrenoceptor. Submitted.
Location
RN Robertson Building #46
NOW Jan Anderson Seminar Room E101A

