PS PhD Exit Seminar - TBA
Hydrogen peroxide (H₂O₂) is a key retrograde signal in plants, linking chloroplast-derived stress cues to nuclear gene expression.
Speakers
Event series
Content navigation
Description
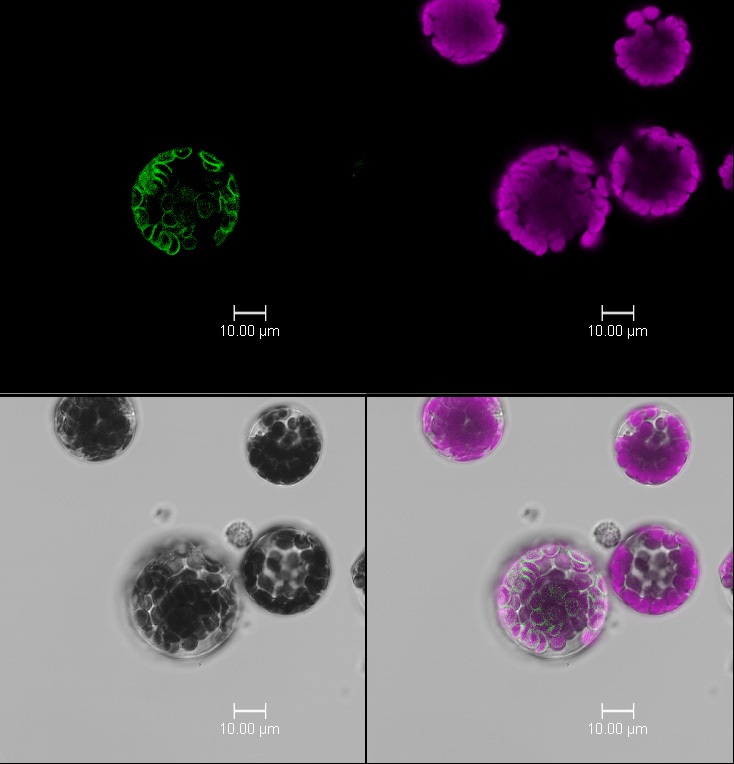
Abstract: Hydrogen peroxide (H₂O₂) is a key retrograde signal in plants, linking chloroplast-derived stress cues to nuclear gene expression. While it plays an important role in modulating plant stress responses, studying H₂O₂ in isolation is challenging due to its overlap with other reactive oxygen species and signaling pathways. Tools that enable spatial and conditional control of H₂O₂ sensing and transport would provide new insight into its function in redox signaling.
My PhD research aims to develop a synthetic biology toolkit to study H₂O₂-mediated retrograde signaling and lay the groundwork for building customisable gene circuits for stress responsiveness. In the first stage of this work, I identified and validated chloroplast outer envelope targeting domains by fusing candidate sequences to mCherry and screening for localization in Arabidopsis protoplasts. This established a platform for spatially precise sensing and signal modulation at the chloroplast surface.
Building on this, I investigated aquaporin-mediated H₂O₂ transport as a strategy for tuning chloroplast-derived redox signals. I engineered the known transporter AtTIP1;1 with chloroplast outer envelope targeting domains and confirmed its functionality through AlphaFold2 modeling, yeast growth assays, and confocal imaging. These results showed that modified AtTIP1;1 retained pore structure and H₂O₂ transport efficiency. I also generated AtTIP1;1 overexpression lines in wild-type and knockout Arabidopsis backgrounds to enable in planta studies of H₂O₂ flux.
The final phase of this project focused on developing cleavage-based output modules responsive to H₂O₂. I repurposed the oxidative stress-induced cleavage of the transcription factor ANAC017 by fusing its cleavage domain to mCherry and tethering it to the chloroplast envelope. In protoplasts, I observed possible cleavage and release of the reporter under exogenous H₂O₂ treatment, indicating synthetic compatibility. In parallel, I evaluated the use of two orthogonal viral proteases, TVMVP and TEVP, for conditional cleavage of synthetic constructs. Co-expression studies demonstrated efficient and specific cleavage with minimal cross-reactivity, supporting their future use in logic-gated synthetic circuits.
Together, this work presents a modular toolkit comprising spatial targeting, transport control, and cleavage-based response elements. These tools enable new strategies for dissecting and reprogramming retrograde signaling and contribute toward future synthetic stress-response networks in plants.
Biography: Natalie completed her Honours in 2021 in the von Caemmerer lab, investigating plasmodesmata formation in C3 and C4 monocot leaves. She began her PhD in 2022, focusing on synthetic biology approaches to study hydrogen peroxide signaling in plants. Her research integrates molecular cloning, imaging, and synthetic gene circuit design to explore and rewire retrograde signaling pathways.
Location
Eucalyptus Seminar Room
S205, Level 2
RN Robertson Building (46)


