PS PhD Exit Seminar - Redesigning phosphoenolpyruvate carboxylase for improved catalysis in C4 photosynthesis
C4 photosynthesis, a carbon concentrating mechanism, evolved as an adaptation to improve photosynthetic CO2 assimilation in terrestrial plants under conditions of low CO2, increased temperatures and varying rainfall patterns.
Speakers
Content navigation
Description
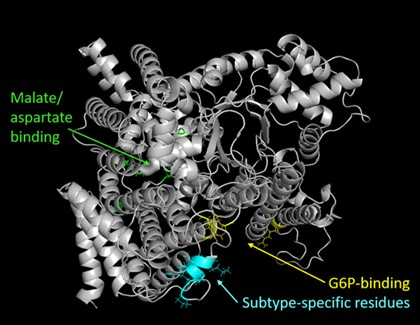
Abstract: C4 photosynthesis, a carbon concentrating mechanism, evolved as an adaptation to improve photosynthetic CO2 assimilation in terrestrial plants under conditions of low CO2, increased temperatures and varying rainfall patterns. Understanding the C4 photosynthetic cycle could prove invaluable when trying to engineer C3 crop species with better photosynthetic rates or to improve the photosynthetic rates of C4 crop species. Phosphoenolpyruvate carboxylase (PEPC), the primary carboxylase of the C4 photosynthetic cycle, operates without any oxygenase activity, and at catalytic rates 3-8 times that of Rubisco. PEPC is responsible for catalysing the irreversible β-carboxylation of PEP by HCO3- to form oxaloacetate (OAA), which is then converted to a 4-carbon carboxylic acid. The identity of this 4C carboxylic acid is dependent on the C4 photosynthetic subtype to which the plant belongs. Regardless of C4 subtype, these organic acids contribute to the cytosolic mesophyll environment, where PEPC is located, and are important in driving the carbon flux to the BSC.
My PhD project consisted of three aims to investigate PEPC:
- Investigate the catalytic properties of PEPC from different species of the Paniceae by recombinant expression in E. coli.
- Modify PEPC enzyme and investigate in vivo effects in the C4 plant, Setaria viridis.
- Investigate effects of increased PEPC expression in the C4 plant, Setaria viridis.
I will focus on the first aim in this exit seminar. A C4 subtype-specific sequence was identified that is proposed to confer differences in PEPC kinetic properties. This sequence, although not directly involved in allosteric regulator binding, affects the inhibition of PEPC by malate. Purification of C4 PEPCs and chimera PEPCs expressed in E. coli, have allowed for a direct comparison between the kinetic properties of different PEPC enzymes and the effect of this C4 subtype-specific sequence on catalysis.
Biography: Rowarne completed her Bachelor of Science at UWA. She completed her Honours at UWA in Martha Ludwig's lab before moving to Canberra to do her PhD at ANU.
Location
Please note: this seminar will be held via Zoom, link below.
Please click the link below to join the webinar:
https://anu.zoom.us/j/82693231920?pwd=YUZsaWFPY1dYbEtHekpaZFNVNGI2QT09
Webinar ID: 826 9323 1920
Passcode: 437732
